Second project period 10/2010 - 03/2015
| Project No. | Project Name | Responsible |
|---|---|---|
| G9 | Rolled-up nanotech for on-chip energy storage | Prof. Dr. Oliver G. SCHMIDT |
Main Objectives
- Develeopment of micrometer-sized electrochemical cells based on a single micro- / nanotube by using rolled-up nanotech
Description
The use of nanomaterials and novel nanotechnologies to create superior circuitries will lead to high performance, low cost and smart nanodevices for a broad range of applications. However, at present most of these nanodevices work with an external macro-sized power source, which presents a painful limitation for any autonomous operation. Although powering nanometer-scale devices has long been a challenge, various approaches have been put forward to construct micro- or nanoscale electrical energy generators, such as coaxial silicon nanowire solar cells and piezoelectric nanogenerators.[G9-1]-[G9-4] These small energy sources are composed of single functional nanostructures, and can greatly reduce the size of integrated nanosystems for wireless and portable nanodevices. Nevertheless, up to now, these sources only operate intermittently, and cannot work without an external energy supply. Therefore, an on-chip micro-/nano- power source including the capability for energy storage is highly desirable.
Although batteries are common energy sources in daily life, the fabrication of micro- or nanoscale electrochemical cells on a chip remains a big challenge. Several issues need to be solved in constructing such a device, including (1) excellent material compatibility of all parts integrated into a small cell, (2) sufficient stability and performance of the applied solidstate electrolytes and electrodes, (3) the capability of chip integration, (4) in depth understanding of interface reactions (e.g. electrolytes vs. electrodes). Very importantly, the power/energy density of the fabricated devices is a crucial issue for micro- or nanoscale electrochemical cells towards any useful applications.
Here, we propose to develop micrometer-sized electrochemical cells (lithium-ion batteries and supercapacitors) based on a single micro-/nanotube by using rolled-up nanotechnology.[G9-5],[G9-6] This device is supposed to work as a steady and rechargeable power source for integrating nanometer-scale functional units on one chip for wireless or portable nano-/microelectronic devices. Electrochemical cells fabricated in such a way are expected to exhibit improved interfaces with well-defined areas, chemical constituents, etc. In particular we will aim at understanding the ionic transport properties across such interfaces in order to improve the overall device quality.
In order to construct rolled-up energy storage device such as batteries or supercapacitors, we deposit multilayer films, which are composed of a sacrificial layer, an insulator layer, a current collector layer (metal), a cathode active layer, an anode active layer and a solid electrolyte layer. After removing the sacrificial layer, the devices will be rolled-up on a chip as schematically shown in Figure G9-1a. Metal electrode pairs will be deposited on the two ends of a selected nano/micro-tube. The electrodes consist of two layers of metal with the outer layer being Au and the bottom layer comprising another metal (Cr, Ti, etc., selected to form a better contact with the tubes). Before rolling up the multilayer, a protection layer will be deposited by atomic layer deposition to prevent any damage from the wet chemical underetching process. After the deposition of electrode pairs, the connection between the rolled-up nano/micro-tube (Fig. G9-1a) and the unrolled planar electrodes on the substrate will be accomplished.
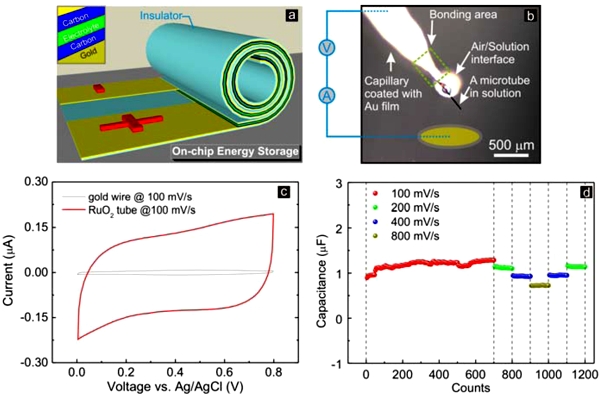
|
|
Fig. 1: (a) Schematic illustration of the proposed structure of a rolled-up microtubebased energy storage device. (b) An individual tube connected to a gold-coated capillary as microelectrode for a supercapacitor; (c) Cyclic voltammetry of a single Au/RuO2 tube in 0.5 M H2SO4 aqueous solution and (d) sustainability of the single microtube-based supercapacitor. |
To examine the feasibility of this concept, an individual Au/RuO2 rolled-up microtube has been tested for use in a supercapacitor. The applied electrochemical method is shown in Fig. G9-1b and presents a clear and typical charge/discharge property in 0.5 M H2SO4 aqueous solution (Fig. G9-1c) compared to only a minor effect observed for a pure Au reference material. The capacitance can reach up to 1.0 µF (200 F g-1) and remains at the same level after thousand cycles using the same scan rate (Fig. G9-1d). These results suggest that a single rolled-up tube can serve as a stable energy storage component in microscale electronic circuits. Up to now, the main disadvantage is the use of a liquid electrolyte. To fabricate on-chip micro/nano-sized power supplies, a solid-state electrolyte (Fig. G9-1a), e.g. LiPON, will be used to separate cathode and anode layers. In this way, an all-solid-state electrochemical cell (lithium-ion battery or supercapacitor) might be achieved using a single multimaterial microtube, eventually powering autonomous micro/nano-scale functional units or other fully integrated rolled-up devices.
Co-operation is planned with G1 (dielectrics coating), G5 (precursors synthesis), G7 (characterization of thin films and interfaces).
References
| [G9-1] | Y. Qin, et al., Nature 451 (2008) 809. |
| [G9-2] | B. Z. Tan, et al. Nature 449 (2007) 885. |
| [G9-3] | X. D. Wang, et al., Science 316 (2007) 102. |
| [G9-4] | Z. L. Wang, et al., Science 312 (2006), 242. |
| [G9-5] | O. G. Schmidt, et al., Nature 410 (2001) 168. |
| [G9-6] | Spange, S.; Kempe, P.; Seifert, A.; Auer, A.A.; Ecorchard, P.; Lang, H:, Falke, M.; Pohlers, A.; Hietschold, M.; Hoyer, W.; Cox, G.; Angew.Chem. (Int. Ed.) accepted |




